Mr H. Lung Cancer
Name of patient
Disease type

Personal story
Mr H is 72, a retired Police Officer of 30 years. He is married, a father of 2, a grandfather to 7 and a great grandfather to 3. He smoked approximately 25 cigarettes/day from the age of 15 for 47 years, quitting in October 2012 with assistance from CamQuit. He had no previous significant illness and no current symptoms.
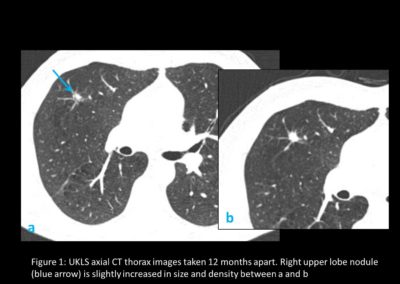
In June 2012, he was invited by UK Lung Cancer Screening Trial (UKLS) to take part in a screening programme. He consented and had his first CT scan on 31/01/2013 and a second on 29/04/2013. The first was clear, the second showed slight changes with a follow-up scan on 17/01/2014.
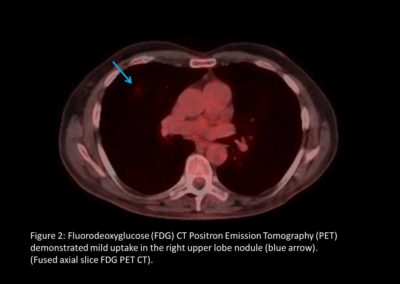
Further scans, investigations and pulmonary function tests were carried out. A CT guided needle biopsy (CTNB) confirmed poorly differentiated squamous cell carcinoma in the right upper lobe. In March 2014, he underwent right VATS upper lobectomy and systemic nodal dissection – pT1aN0Mx of which excision appeared complete.
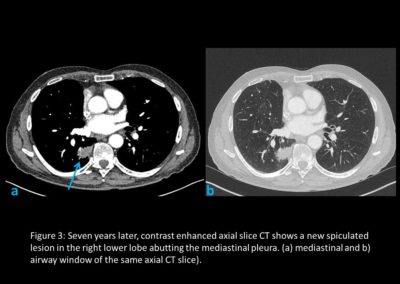
A regular nurse-led clinic follow-up with CXR continued. In February 2021, Mr H enrolled in the Second Primary Lung Cancer Cohort Study (SPORT) Trial.
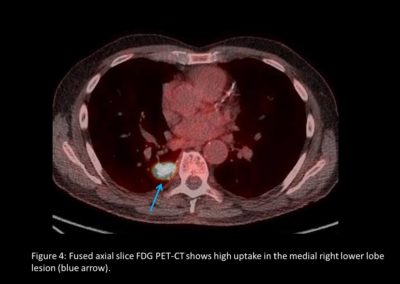
In November 2021, he experienced some shortness of breath and minor heart palpitations. Various cardiac and respiratory investigations were carried out leading on to CTPA and staging CT scans in January 2022, which revealed an abnormality in the right lower lobe (RLL). A further PET-CT confirmed a T2a N0/2 M0 RLL mass. In February 2022, EBUS 7, 11R and EUS 7 confirmed no lymph node involvement. A second CTNB showed squamous cell carcinoma RLL with an additional more atypical spindled and giant cell component. It was not possible to ascertain whether the two tumours were independent primaries or a late recurrence.
In March 2022, a RLL apical wedge resection, converted right antero-lateral thoracotomy and lymphadenectomy performed pT2aN0, PL1 R0 pleomorphic carcinoma, PD-L1 10-20%. Likely 2 primary lung cancers with close monitoring ahead.
Radiological Findings
Almost three-quarters of people with lung cancer present with advanced stage disease. Screening using low-dose CT (LDCT) was shown to reduce lung cancer mortality by 20% in the National Lung Screening Trial (1).
The pilot UK Lung Cancer Screening (UKLS) was a randomised controlled trial of LDCT screening for lung cancer versus usual care (2). It used the Liverpool Lung Project (LLP) risk model, based on a case–control study (3). This is a multivariable conditional logistic regression model based on factors significantly associated with lung cancer (smoking duration, prior diagnosis of pneumonia, occupational exposure to asbestos, prior diagnosis of malignant tumour and early onset (<60 years) family history of lung cancer).2 It includes all respiratory disease and all smokers to select subjects with ≥5% risk of developing lung cancer in the following 5 years (risk prediction model http://www.MylungRisk.org). Inclusion required a 5-year lung cancer risk of ≥5% in men and women aged between 50 and 75 years. CT screen-detected nodules were managed by a pre-specified protocol (Figure 1).
As part of the National Optimal Lung Cancer Pathway (NOLCP) (4), PET-CT is now a standard of care prior to surgery / radical treatment, to aid biopsy decision and staging of tumour (Figure 2 and Figure 4). Following surgery, the importance of protocol-led or research driven follow-up is essential to detect recurrence or the possibility of a further new primary (Figure 3) (5,6). Clinical trials must be supported, promoted and become part of patient culture at all points in their pathway.
Lavinia Magee, Nurse Consultant Thoracic Oncology (LCNUK, LuCE)
Dr A Barker, Consultant Radiologist
Royal Papworth Hospital, UK
References
- Aberle DR, Adams AM, Berg CD, et al. , National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. 10.1056/NEJMoa1102873
- Field JK, Vulkan D, Davies MPA, et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg Health Eur. 2021 Sep 11;10:100179. doi: 10.1016/j.lanepe.2021.100179. PMID: 34806061; PMCID: PMC8589726.
- Cassidy A, Myles JP, van Tongeren M, et al. . The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270–6. 10.1038/sj.bjc.6604158
- National optimal Lung Cancer Pathway (NOLCP) http://content.smallerearthtech.co.uk/system/file_uploads/16086/original/National_Optimal_LUNG_Pathway_Aug_2017.pdf