Urbain Bruggeman Prostate Cancer
Name of patient
Disease type

Personal story
July 2016, Urbain undergoes an MRI to find the cause(s) of an important decrease of his mobility in the pelvic area. The MRI reveals suspicious spots on the bones. Further testing by the urologist, a PSA level of 23µg/L, a Gleason score of 9 (5+4) and alkaline phosphatase over 300 IU, reveals metastasized aggressive prostate cancer. A subsequent bone scan confirms the metastasis.
Monthly medication with Firmagon and six sessions of chemotherapy result by the end of 2016 in a PSA of 0,01µg/L and normal alkaline phosphatase. Treatment with Firmagon is continued.
At the end on 2017 his PSA is rising again to 0,02µg/L and further increases, to double in less than three months in the spring of 2020.
A PSMA-PET-CT scan reveals a new tumour in his prostate. The treatment is adjusted by adding daily abiraterone-acetate (and prednisolone). Each month Urbain receives an injection with Xgeva to prevent bone loss and finally Zoladex replaces Firmagon.
In a few months’ time his PSA level decreases again to a level of 0,02µg/L to rise slowly to a level of 0,10µg/L today.
His cancer is monitored with six monthly CT scans and a yearly bone scan. Up to today his prostate cancer is calm and asleep.
Although his prostate cancer was (and is) very aggressive, he never had pain. This makes his life and quality of life positive up to today, but this requires obeying a strict disciplined life style.
Radiological Findings
In prostate cancer (PCa), 20-50% of patients will develop biochemical recurrence within 10 years after primary treatment with curative intent (radical prostatectomy / radiation therapy). To individually tailor treatment options, sensitive as well as specific imaging is needed to identify the culprit lesions. Prostate specific membrane antigen (PSMA) is a cell-surface glycoprotein overexpressed on PCa cells. At present, PET/CT with PSMA-ligands is the gold standard imaging modality in patients with biochemically recurrent PCa [1] for 2 main reasons: (1) PSMA PET/CT can accurately localize disease in patients with biochemically recurrent PCa. A prospective multicentre trial (n=635) with 68Ga-PSMA-11 PET/CT in PCa patients with biochemical recurrence, revealed a positive predictive value of 84% and sensitivity of 92% of 68Ga-PSMA-11 PET in detecting recurrence by histopathologic validation and (2) PSMA PET/CT successfully guides treatment in patients with biochemically recurrent PCa. 68Ga-PSMA-11 PET/CT-directed focal therapy alone leads to a PSA decrease of 50% in 80% of the patients [2].
This robust data has led to the investigation of the role of PSMA PET in the primary staging of PCa. In the proPSMA trial, a randomized controlled trial studying 68Ga-PSMA-11 PET/CT in high-risk PCa before curative-intent surgery or radiotherapy, PSMA PET/CT had a 27% greater accuracy than bone scintigraphy and abdominopelvic CT for detecting nodal or distant metastases [3].
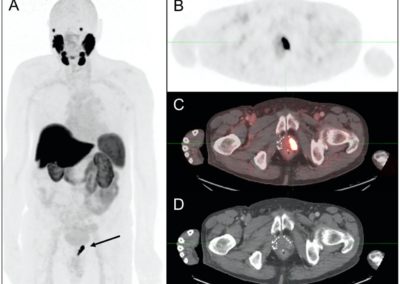
Figure: 18F-PSMA-1007 PET/CT in biochemically recurrent PCa. Patient had received previous brachytherapy and developed rising PSA (5.2 ng/ml) 14 years later. (A) Maximum intensity projection, (B) axial PET slice, (C) axial PET/CT fusion slice and (D) axial CT slice show intense PSMA-uptake in a local recurrence in the left basolateral prostate segment (wide arrow). Patient received salvage local treatment.
Prof. Dr. Karolien Goffin, Nuclear Medicine and Molecular Imaging, University Hospital Leuven and KU Leuven, Leuven, Belgium
References
- Mottet N. EAU-ESTRO-SIOG guidelines on prostate cancer. EAU Annual Congress. Barcelona; 2021
- Fendler WP, Calais J, Eiber M, Flavell RR, Mishoe A, Feng FY, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer: A Prospective Single-Arm Clinical Trial. JAMA Oncol. 2019;5:856-63.
- Hofman MS, Lawrentschuk N, Francis RJ, Tang C, Vela I, Thomas P, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208-16.